Technologies
Chemistries
A broad range of lithium-ion cell chemistries may be found on the market, as several possibilities exist for the choice of cathode material. The anode material is generally carbon/silicon and graphite (LTO is the exception). The ability to drain high currents, the internal resistance, or some more global characteristics such as the power density will be chemistry-sensitive. Finding out the main chemistry of a cell is not always an easy task, as manufacturers continuously improve their technologies and may change the naming conventions for commercial purposes. However, the main categories are summarized in the table below.
Name | Chemical abbreviation | Cathode | Anode | Convention in PVsyst | Other convention | Nominal Voltage [V] | Power density [Wh/Kg] |
|---|---|---|---|---|---|---|---|
| Lithium Titanate | Li4Ti5O12 | Graphite | Li4Ti5O12 | LTO | 2.0 | 80 | |
| Lithium Iron Phosphate | LiFePo4 | LiFePo4 | Graphite | LFP | IFR | 3.2 | 120 |
| Lithium Manganese Oxide | LiMn2O4 | LiMn2O4 | Graphite | LMO | IMR | 3.6 | 140 |
| Lithium Manganese Nickel | LiNiMnCoO2 | LiNiMnCoO2 | Graphite | NMC | INR | 3.4 | 200 |
| Lithium Cobalt Oxide | LiCoO2 | LiCoO2 | Graphite | LCO | ICR | 3.7 | 200 |
| Lithium Nickel Cobalt Aluminum Oxide | LiNiCoAl2 | LiNiCoAl2 | Graphite | NCA | NCR | 3.6 | 250 |
One can distinguish 2 main categories for cells, Energy Cells (high capacity expected) or Power Cells (high drawn currents expected). The chemistry will generally be selected according to the cell usage. A chemistry is always a balance between energy, capacity, cycle life and safety. Even if chemical reactions are often complex phenomena, here is the general picture :
- Phosphate brings safety and long cycle life.
- Manganese lowers the internal resistance. This allows the cell to be discharged at low temperature and high current.
- Nickel brings capacity.
- Cobalt brings capacity, but at a cost and with safety concerns.
The LFP category is popular for stand alone solar applications due to its enhanced safety, when the power density is not the most important criteria. They successfully replace lead-acid batteries.
For residential applications, where available space can be an issue, chemistries such as NCA may be preferred. The NMC chemistry shows a good balance of properties and covers a wide range of applications.
Lithium Polymer cells use a polymer gel as an electrolyte, whereas all other cells have a liquid electrolyte.
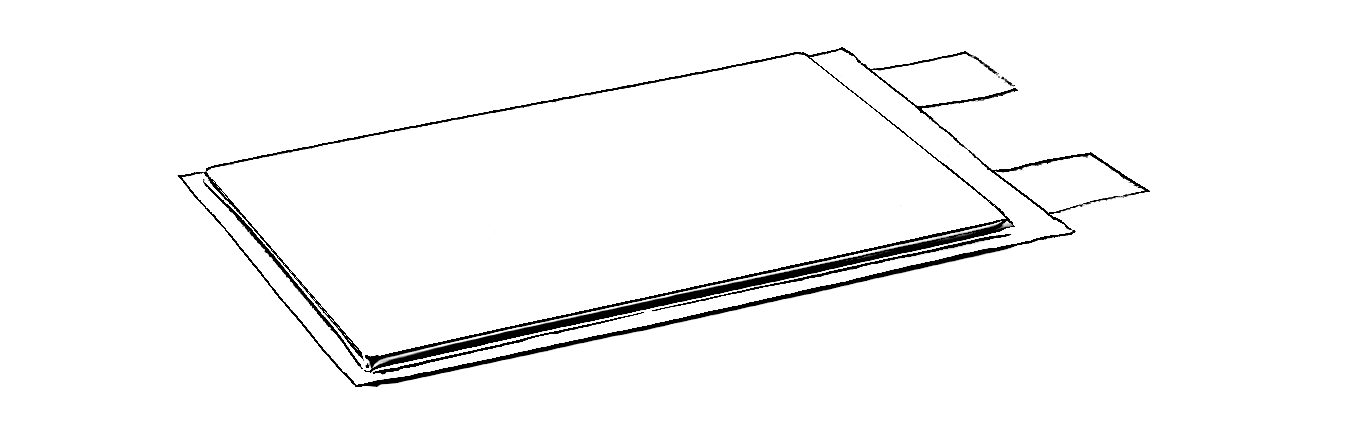
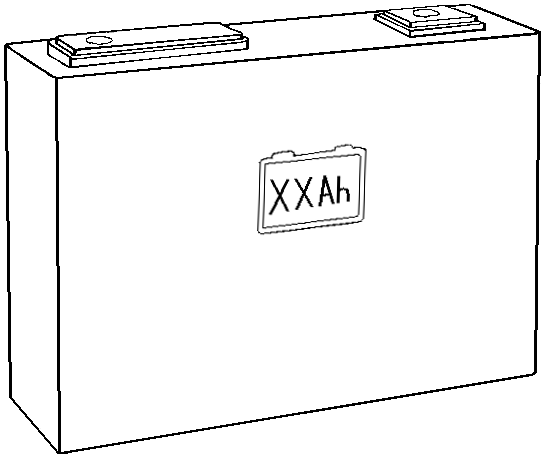
Shapes
Lithium-ion cells may be manufactured as cylindrical cells, pouch cells, or prismatic cells.
- Cylindrical cells are identified by a 5 digit number, which refers to the cell dimension. The last digit,0, stands for "cylindrical". There are 2 popular references :
- the 18650 cell : diameter 18mm, length 65mm, 47 grams.
- the 26650 cell : diameter 28mm, length 65mm , 82 grams.
- Pouch cells are flexible designs with a parallelipipedic shape. They cannot be used as such as they do not retain their shape during usage.
- Prismatic cells are rigid designs with a parallelipipedic shape.
Cylindrical cells are produced at low cost but have a lower volumetric efficiency compared to pouch cells and prismatic cells, which, in return, are produced at a higher cost. A continuous effort is done by manufacturer to evacuate the heat, as this is a key point to build efficient and safe systems.
| Cylindrical cell | Pouch cell | Prismatic cell |
|---|---|---|
 |  |  |