Battery model justification
Lead-Acid batteries
We have given up to use the classical models (for example Shepherd's model1), where a number of parameters are involved, which require practically a detailed measurement for each battery model used.
We have tried to develop a rather simple model, which should reproduce the fundamental data furnished by all constructors, but based on information present on usual datasheets.
We have added some specific disturbances, not specified on the datasheets. When establishing the model, we had close contacts with the technical service of a manufacturer. He gave some tips and specific data, which are supposed to be of rather general validity for all Lead-Acid batteries, as based on the same chemistry. For example the value and temperature behavior of the self-discharge, the capacity temperature dependency, etc. Other articles were also of great help 2, 3, 4, 5.
For these secondary behaviors, when unknown, the user can do with the default values, specific to each type of technology, and proposed by the software.
Li-Ion batteries
The Li-Ion battery operating parameters and performance are not public. When establishing the model, very few battery manufacturers accepted to share detailed operating data of there products, and this was usually confidential.
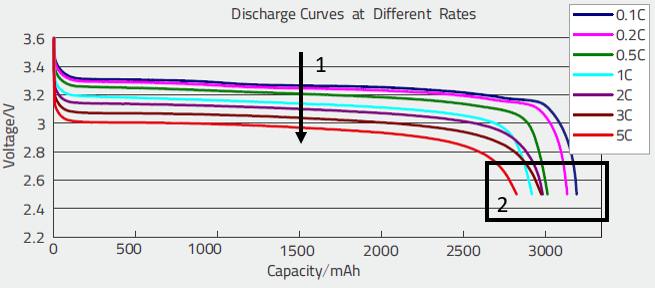
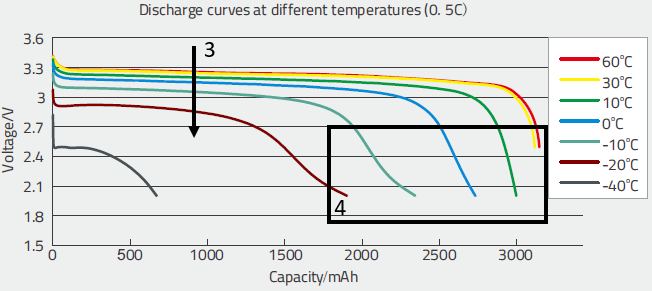
We tried to establish a model which reproduces at best the behavior of the curves we could obtain. In fact the model developed for the Lead-Acid batteries could be adapted without major problem. The parameters were adjusted to match the sets of curves, like these voltage discharge curves (and possible other ones, not shown here.)
These curves allowed to evaluate:
- The basic voltage at SOC = 0.5, and slope as a function of the SOC, for the evaluation of VocBattery.
- The behavior at high SOC (overcharging) and deep discharge,
- The drop of voltage as a function of the discharge rate (1), which allows namely to evaluate the internal resistance,
- The diminution of the capacity as a function of the discharge rate (2); this allows to evaluate the parameter of the Peukert model.
- The diminution of the voltage when the temperature decreases (3), this shows that the variation becomes more significant below 20°C,
- The huge diminution of the capacity with low temperatures (4). Again, the effect is negligible above 30°C and becomes significant below 20°C.
All of these parameters may be different for different chemistries, but we admitted that they remain rather homogeneous within a same technology. We did not obtain recent detailed data (this is probably more and more confidential), and we usually apply the same model for secondary parameters with modern batteries, for which we have usually very limited information.
Therefore when defining a new battery, we use the parameters of an existing cell of the same technology, and modify only the parameters available in the datasheets.
-
C .M. Shepherd
Design of Primary and Secondary Cells.
and:
An equation describing Battery Discharge.
Journal of the Electrochemical Society, vol 112(7), July 1965, pp 657-664. ↩ -
Watsun-PV - User's Manual and Program Documentation
Watsun Simulation Laboratory, University of Waterloo, Waterloo, Ontario N2L 3G1, 1992. ↩ -
Bopp, Gabler, Sauer, Jossen, Höhe, Mittermeier, Bächler,Sprau, Willer, Wollny.
A Systematic Effort de Define Evaluation and Performance Parameters and Criteria for Lead-acid Batteries in PV Systems.
13th European Photovoltaic Solar Energy Conference, Nice, Oct 1995. ↩ -
J.B. Copetti, F. Chenlo and E. Lorenzo
Comparison between Charge and Discharge Battery Models and Real Data for PV Applications.
11th European Photovoltaic Solar Energy Conference, Montreux, Oct 1992, pp1131-1134. ↩ -
J.B. Copetti, F. Chenlo
Internal Resistance Characterization of Lead-Acid Batteries for PV Rates
11th European Photovoltaic Solar Energy Conference, Montreux, Oct 1992, pp1116-1119. ↩